Container Closure Integrity
Tester:
LT-5000 CCIT
“Electronic + Compliance + Equivalence”
The LT-5000 CCIT is a precise leak detection system used to verify the seal integrity of containers holding injectable drugs and other rigid packaging. It can consistently find very small leaks to maintain quality and safety standards.
Comply with ASTM, FDA 21 CFR Part 11, USP 1207, NABL, CE, ISO 9001:2008, EMA ANNEX 11, ProdNx,
Advantages
- No sample damage or prep required; zero operator bias
- Detects defects as tiny as 0.005 ccm
- Identifies leaks of sizes up to 0.5 µm
- More sensitive and consistent than dye ingress testing
- Offers quantitative, deterministic results
- Complies with 21 CFR Part 11, ASTM, and FDA standards
- Fast, reliable for rigid/flexible packaging. Test time: Settable
- Easy-to-use, touch-screen, compact design
Applicability
- Vacuum decay leak testing is a non-destructive method for verifying container closure integrity (CCI) by detecting leaks from large to micron-sized in pharma, medical device, and food packaging.
- Recognized by ASTM F2338-09 and PDA as a standard for non-destructive leak detection.
- Batch testing
- For new product set-up, test & development
- Production lines
- Non-destructive testing
- Bacteria-free ingress test
Technical Specifications
| SPECIFICATIONS | MICRO LEAK DETECTION | CONTAINER CLOSURE INTEGRITY TESTING |
|---|---|---|
| Packaging Format |
|
|
| Testing Setup | Offline laboratory and production line applications | |
| Test Method | Vacuum/Pressure decay method | |
| Inspection Platform | Dual Transducer Technology | |
| Display Screen | 7"/10" color touch screen - resistive/capacitive | |
| Standard Procedure | ASTM F2338-09 | |
| Accuracy | Up to 0.5 micron | |
| Measurement Unit & Limits | Pass/Fail in mBar & Pascal units | |
| Sample Feeding | Manual/Automatic | |
| Housing | ABS/SS | |
| Size | 14.96” W × 10.23” D × 10.31” H | |
| Weight | 14kg | |
| Electrical | 100–240 VAC; 50/60 Hz | |
| Input Air Supply | External/in-built (optional) | |
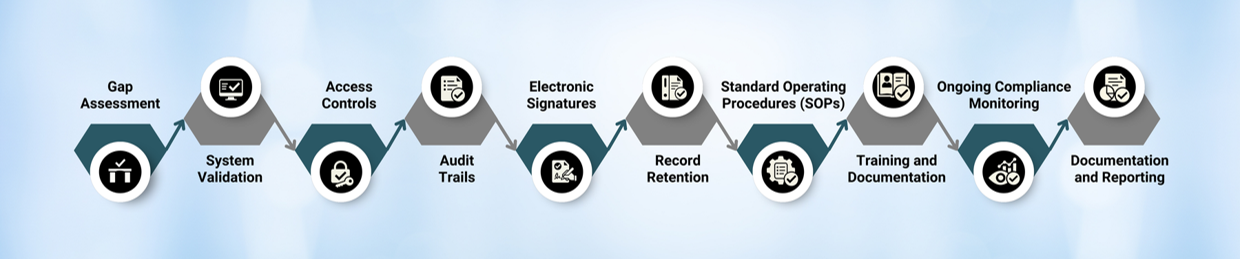
21 CFR PART 11 COMPLIANT LT-5000 CCIT
- To keep pace with the increasing use of electronic systems and technology in FDA-regulated industries, 21 CFR Part 11 was introduced, ensuring the same level of data integrity, authenticity, and reliability as paper-based systems.
- Basically, 21 CFR Part 11 is a regulation established by the U.S. Food and Drug Administration (FDA) that outlines the requirements for the use of electronic records and electronic signatures in FDA-regulated industries, including pharmaceuticals.
- Adhering to these guidelines, the introduction of Information Technology (IT) in compliance was a milestone.
- It establishes requirements for the security, integrity, and availability of electronic records and the validation of electronic systems used for GxP (Good Laboratory Practices, Good Clinical Practices, Good Manufacturing Practices) activities.
Audit Trail
- Tamperproof & Non-Editable Audit Trail Data Format.
- The time stamp of the change of the parameter value & the user making the change.
- The audit trail records the following
details
- User Creation
- User Login/Logout
- Wrong attempts at login
- User Block / Unblock by Administrator
- Old value & new value of the parameter change
- The time stamp of each event
Electronic Signatures
- Through 3/4 user level validation & authentication
System Data & Data Backup
- Through OPC UA
- Through Data file transfer
- Through USB / SD Card Backup
HMI offers basic connectivity for data exchange with Central SCADA / MES / ERP by following the means:
Electronic Data Record & Data Storage
- Review of the reports on the HMI Screen for Production, Alarms, & Audit Trail.
- Storage limit can be interlocked in terms of the number of batches produced or % of the memory consumed.
User Management Functionalities
- Password-protected individual Unique user account.
- Password Complexity.
- Minimum 8 Character password length.
- Configurable Number of Wrong Attempts.
- User Block/Unblock Facility.
- Password Validity.
- Multiple User Levels as per the User Rights.
Report Generation & Printing
- Batch data is stored in a secure database format, & the Batch Report can be generated using this data.
- The report printing has been offered in multiple ways.
- Online printing of Alarms, Events, & Logged data through Serial Printer.
STEP FOR IMPLEMENTATION OF 21 CFR PART 11
Applications
Vials

Ampules

Pharma Bags
Accessories
- Vacuum Pump
- Micro calibrated leak
- Test Cavity
- Master (Solid dummy)
- Sealing and Connectors
- Barcode Printer
- Barcode Scanner
- External Start/Stop Pendant
- Micro drilling services
News from Infinity Automation
Trade fairs & Events
Contact us
Headoffice
Would you like to contact Infinity
Automation online?
Then use our online contact form!
.png)






